It was well known that dietary di/tripeptides elicit preventive effects against lifestyle-related diseases such as hypertension, and hypercholesterolemia, etc. Although there have been evidential reports that the intake of protein hydrolysate improved impaired memory in humans, limited studies on bioavailability, in particular, beyond the blood-brain barrier (BBB) of candidates in hydrolysate may prevent their extensive physiological studies (Figure 1). Thus, this review discusses the updated studies on BBB transport of peptides showing improved cognitive decline. Furthermore, their accumulation in the brain cerebral parenchyma is also introduced.
    |
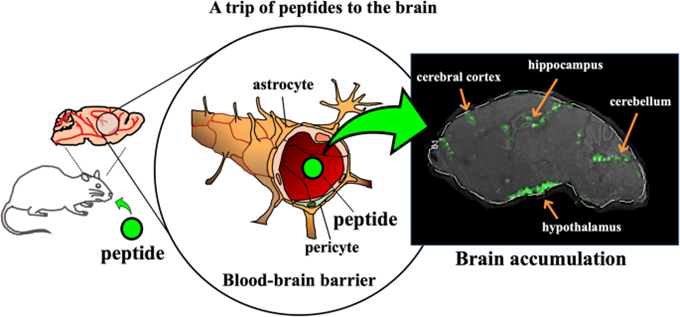 |
| Figure 1. A trip of peptides to the brain |
The physiological role of peptides is deemed important from the aspect of preventive lifestyle-related diseases, such as hypertension, diabetes, and atherosclerosis. Clinical evidences prove their effective health benefits in humans, for example, the anti-hypertensive effect shown by daily intake of small (di/tri) peptides, which modulate promoting blood pressure. The anti-hypertensive effect of peptides (e.g., Val-Tyr) is closely associated with the suppression of promoting systemic and local renin-angiotensin (RA) systems by inhibiting angiotensin I-converting enzyme (ACE) activity. In addition, the suppression of local RA systems by peptides in the aorta and kidney extensively leads many researchers to study the potential of peptides in local organs. Some researchers have clarified the action of diverse peptides in improving degraded vessel functions.
Many researchers have illustrated that many small peptides are located in diverse organs including the intestines, kidneys, spleen, liver, lungs, and brain. In contrast, their characteristics on peptide recognition at each organ/transporter remain unclear. In particular, the biggest challenge for peptide transport is to account for the role of transporters expressed in the brain because peptide transporters expressed in the brain endothelial cells surrounded by pericytes, astrocyte, and neurons play a crucial role in the efflux (clearance) of peptidic metabolites from the cerebrospinal fluid. This review will discuss the possibility of peptide transport through the blood-brain barrier (BBB). Contribution of the preventive potential of peptides on brain functions is also reviewed from the point of view of memory and cognitive impairment.
Transportability of peptides across the BBB
Several assays, such as in vitro primary cell membrane, ex vivo sliced brain tissues or ventricle plexus, and in vivo brain perfusion experiments, have been performed for the evaluation of BBB transportability or uptake of compounds in the direction of blood to brain. The in vitro transport (influx) assays of targets comprise a transwell chamber system with cells grown into the insert membrane. Carnosine (β-Ala-His, Pro-Pro-Leu, and cyclo (Phe-Phe) were reported to be transportable by the cell line experiments. Therefore, in vivo transport experiments by target perfusion in mouse must provide substantial evidence to solve the mystery whether peptides can cross the BBB and accumulate into the cerebral parenchyma. In mouse treated by ligation of the thoracic aorta, a perfusion solution containing targets is infused in the left of ventricle of the heart for a few minutes (Figure 2).
    |
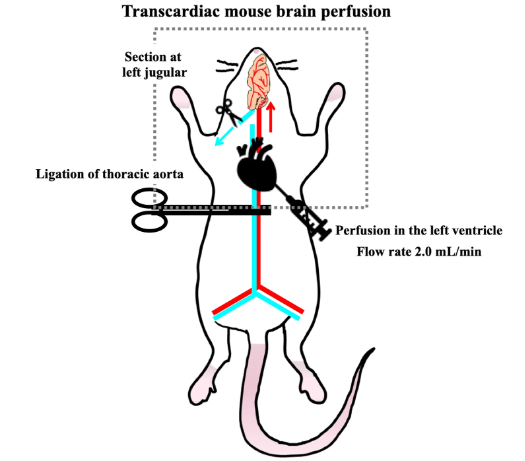 |
| Figure 2. Transportability of peptides across the BBB |
Blood-to-brain transporting peptides:
To explain whether peptides are of benefit for brain functions, in vivo evidence on peptides capable of influx transporting beyond the BBB must be provided prior to comparative study on transportability by in vitro cell line transport experiments, considering above issues regarding protease degradation and highly integrated barrier of the BBB. Thus, in situ perfusion experiments provide in vivo evidence on possible transportability of peptides in intact form across the BBB (Figure 3).
    |
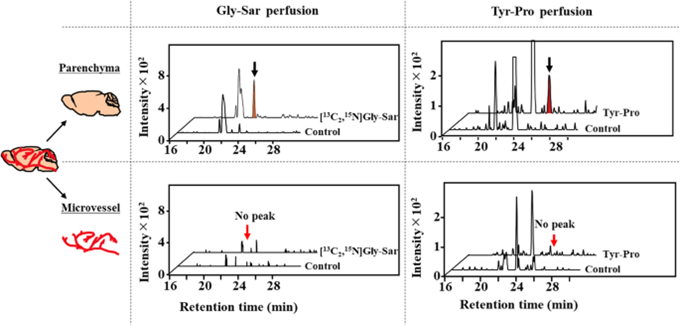 |
| Figure 3. Transport of dipeptides, Gly-Sar and Tyr-Pro, into the parenchyma of 2.0-min perfused mouse brain. [13C2,15N]Gly-Sar and Tyr-Pro in the parenchyma and microvessel fractions of mouse brain were detected by LC-TOF/MS |
Location of peptides in the brain
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) imaging technique is an extensive and advanced visualization tool having wide applications in spatial localization of targets in terms of tissue distribution, such as immunostaining. This is because it possesses the great advantage of non-targeting detection for a wide mass range of ionizable compounds such as proteins, lipids, and drug molecules with no use of antigens. The novelty of the phytic acid-aided MALDI-MS imaging technique could be applied for the histological distribution of Tyr-Pro capable of crossing the BBB in intact form (Figure 4). As depicted in Figure 4, it seems probable that Tyr-Pro may preferably accumulate in the hippocampus, hypothalamus, striatum, cerebral cortex, and cerebellum of the mouse brain after < 10 min-perfusion. The accumulated regions of Tyr-Pro in the mouse brain are closely associated with memory consolidation and spatial memory (hippocampus), food intake control (hypothalamus), and cognition (cerebrum and cerebellum). The accumulation of BBB transporting dipeptides in brain parenchyma is unknown because there have been no comparable reports so far. However, the evidential observation by MALDI-MS imaging could indicate a potential physiological function of dipeptides in the brain.
Figure 4. Visualized distribution of Tyr-Pro in perfused mouse brain by MALDI-MS imaging. MALDI-MS/MS images of Tyr-Pro ([M + H]+, 279.1 > 116.0 m/z) display the sagittal tissue and coronal sections of a 10-min Tyr-Pro-perfused mouse brain. Scale bar, 2 mm; spatial resolution, 90 μm
Conclusion
It seems likely that some dipeptides may cross the severely controlled barrier, BBB, and may accumulate in the brain's cerebral parenchyma in intact form. Animal and human studies also provide their brain health benefit against cognitive decline. However, we still need studies to clarify the underlying mechanism(s) and to see if the benefit carries over to humans.
Souce: https://fppn.biomedcentral.com/
Vu Thi Hanh, Faculty of Food Science and Technology