Cordyceps militaris, a highly valued medicinal mushroom, is known for its numerous health benefits. However, cultivation of this fungus faces a significant challenge: strain degeneration. This phenomenon leads to decreased yield and quality of the final product. Among the factors contributing to degeneration, the MAT (Mating-type) gene system is considered to play a critical role. This paper aims to analyze the role of the MAT system in the degeneration of Cordyceps militaris strains.
The MAT gene system governs mating and genetic recombination in fungi, influencing their sexual reproduction and life cycle. In C. militaris, the MAT system consists of two primary locus: MAT1-1 and MAT1-2. The MAT1-1 locus contains two genes, MAT1-1-1 and MAT1-1-2, while the MAT1-2 locus harbors only one gene, MAT1-2-1. These genes encode transcription factors that regulate the expression of other genes, ultimately affecting fruiting body development.
The Relationship Between the MAT System and Strain Degeneration:
Studies have established a strong correlation between the MAT gene system and strain degeneration in C. militaris. Some key findings are:
- Loss or Alteration of MAT Genes: Degenerated strains frequently exhibit a loss or alteration in the structure of MAT genes. For instance, research has indicated that certain degenerated C. militaris strains have lost the MAT1-2-1 gene segment and display point mutations within the MAT1-1-1 and MAT1-1-2 gene regions.
- Shifts in MAT Gene Ratios: Strains exhibiting lower ratios of MAT gene-carrying spores tend to experience a higher degree of degeneration. For example, research reveals that the S1 strain, possessing the highest ratio of MAT gene-carrying spores (68.57%), also demonstrates the most robust growth, development, and fruiting body production. Conversely, severely degenerated strains (S5, S6) lack any detectable MAT gene components.
- Impact on Fruiting Body Formation: Variations within the MAT system can affect the ability of C. militaris to form fruiting bodies. Research suggests that strains carrying both the MAT1-1-1 and MAT1-2-1 genes can produce complete fruiting bodies. In contrast, strains harboring only a single MAT1-1 or MAT1-2-1 gene may generate fruiting bodies incapable of sporulation or fail to produce fruiting bodies altogether. However, contradictory observations have also been reported, highlighting the complex relationship between MAT genes and fruiting body development in C. militaris.
    |
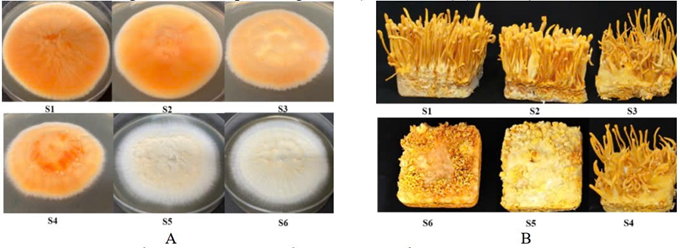 |
| Figure 1. The development of mycelium and fruiting bodies of different Cordyceps militaris strains. A: Morphology of 6 strains on primary inoculation medium; B: Morphology of fruiting bodies after 60 days of cultivation on substrate; S1: Cordyceps militaris strain S1; S2: Cordyceps militaris strain S2; S3: Cordyceps militaris strain S3; S4: Cordyceps militaris strain S4; S5: Cordyceps militaris strain S5; S6: Cordyceps militaris strain S6 [2]. |
Explaining Strain Degeneration Linked to the MAT System:
While the precise mechanism of strain degeneration in C. militaris remains incompletely understood, several hypotheses have been proposed to elucidate the connection between the MAT system and this phenomenon:
- Imbalanced Gene Expression: Loss or alteration of MAT genes can lead to imbalances in the expression of genes associated with growth, development, and fruiting body formation. This imbalance could stem from the dysfunction of transcription factors encoded by MAT genes or from the aberrant activity of affected target genes.
- Limited Genetic Recombination: Loss or alteration of MAT genes may restrict genetic recombination capabilities, resulting in the accumulation of deleterious mutations and a decline in the fungus's adaptability.
- Influence on Natural Selection: In natural environments, the MAT system ensures genetic diversity through random mating. However, under artificial cultivation conditions, artificial selection and asexual propagation practices can diminish genetic diversity. This reduction can inadvertently favor the prevalence of strains carrying disadvantageous MAT genes, ultimately leading to strain degeneration.
Conclusion:
The MAT mating-type gene system plays a crucial role in the reproduction and development of C. militaris. Variations within this system have been demonstrably linked to the phenomenon of strain degeneration. A deeper understanding of the MAT system's role in this context is paramount for developing effective strategies to control strain degeneration, thereby enhancing the yield and quality of Cordyceps militaris in the future.
Reference:
1. Shrestha, B., Zhang, W., Zhang, Y., & Liu, X. (2012). The medicinal fungus Cordyceps militaris: research and development. Mycology, 3(3), 168-172.
2. Dong, T. T., Phan, V. T., & Le, V. V. (2022). Phân lập và xác định một số đặc điểm sinh học của nấm đông trùng hạ thảo (Cordyceps militaris) phân lập tại Hà Nội. Tạp chí Khoa học, Công nghệ và Môi trường, 13(4), 172-179.
3. Zheng, P., Xia, Y., Xiao, G., Huang, L., Liu, X., & Zhang, L. (2011). Complete mitochondrial genome sequence of the medicinal mushroom Cordyceps militaris and its phylogenetic relationship to other Cordyceps species. Journal of microbiology, 49(2), 323-329.
4. Holliday, J. (2004). Early studies on sexual reproduction in the human fungal pathogen Aspergillus nidulans. Fungal Genetics and Biology, 41(3), 150-156.
5. Kück, U., & Pöggeler, S. (2003). MAT, Mating-type loci of heterothallic fungi. In The Mycota (pp. 131-147). Springer Berlin Heidelberg.
6. De Mattos-Shipley, K., Ford, R., Alberti, F., & Banks, M. A. (2010). Identification of a mating-type gene in the asexual fungal pathogen Aspergillus fumigatus. PLoS pathogens, 6(9), e1001074.
7. Dyer, P. S. (2007). Sexual reproduction in Aspergillus fumigatus: a fascinating story. Medical Mycology, 45(Suppl 1), S79-S83.
8. Gioti, A., Mushegian, A. A., & Strandberg, R. (2012). Unidirectional evolutionary transitions in fungal mating systems and the role of transposable elements. Molecular biology and evolution, 29(11), 3215-3225.
9. Lee, S. C., Li, E. K., St Leger, R. J., Roberts, D. W., & Bidochka, M. J. (2010). Dormant sex is downregulated by evolutionarily recent transposons in a fungal plant pathogen. PLoS pathogens, 6(1), e1000748.
10. Ohm, R. A., de Jong, J. F., Lugones, L. G., Aerts, A., Kothe, E., Stajich, J. E., ... & Grigoriev, I. V. (2010). Genome sequence of the model mushroom Schizophyllum commune. Nature biotechnology, 28(9), 957-963.
11. Yun, S. H., Kim, J. Y., Lee, S., Park, J. M., Roberts, D. W., & Lee, S. (2015). Genome-wide analysis of the mating-type locus genes in Fusarium graminearum. Fungal Genetics and Biology, 81, 1-9.
12. Coppin, E., Debuchy, R., Arnaise, S., & Picard, M. (1997). Mating types and sexual development in filamentous ascomycetes. Microbiology and Molecular Biology Reviews, 61(4), 411-428.
13. Idnurm, A., Walton, F. J., Floyd, A., & Heitman, J. (2010). Identification of the sex genes in an early diverged fungus. Nature, 464(7287), 517-520.
14. Fraser, J. A., Diezmann, S., Subaran, R. L., Allen, A., Lengeler, K. B., Dietrich, F. S., & Heitman, J. (2004). Convergent evolution of chromosomal sex-determining regions in the animal and fungal kingdoms. PLoS Biology, 2(12), e384.