In modern society, lifestyle-related diseases concomitant with chronic diseases, such as atherosclerosis, heart disease, stroke, obesity, and type 2 diabetes, have been rapidly increased as a critical public health issue in the world [1]. It is estimated that there are approximately 60 million deaths worldwide each year, in which over half are related to lifestyle-related diseases. The classes of diseases can be improved by lifestyle changes and early treatments such as healthy diet, non-smoking, reducing excessive alcohol use, reducing stress level, and regular exercise [2].
It is well known that a healthy diet plays an important role in disease prevention or modulation. For this reason, food scientists have researched the physiological activities of food compounds, in particular, bioactive peptides from food proteins, which can exert positive physiological responses in the body upon their basic nutritional compositions in the provision of nitrogen and essential amino acids [3]. It has been demonstrated that bioactive peptides are essential in the prevention of lifestyle-related diseases such as hypertension [4–8], antioxidation [9], and inflammation [3]. Thus far, many peptides with various bioactive functions have been discovered and identified [9–12]. It was known that peptides generally consisting of 2 to 9 amino acids may elicit bioactivities [5,9]. Among them, small peptides showing antihypertensive activity by angiotensin-converting enzyme (ACE) inhibition, renin inhibition, and calcium channel blocking effects are in common [13].
The source of food-derived bioactive peptides is mainly from dietary proteins (milk, meat, egg, and soybean) [6,9,14–16]. So far reported, Sipola et al. [17] demonstrated that a long-term administration (12 weeks) of peptides (Ile-Pro-Pro and Val-Pro-Pro) or sour milk containing both tripeptides to 12- and 20-wk spontaneously hypertensive rats (SHR) resulted in a significant decrease in systolic blood pressure (SBP) of 12 or 17 mmHg, respectively. A dipeptide, Val-Tyr, from sardine muscle hydrolysate, showed a significant clinical antihypertensive effect in mild hypertensive subjects [6]. Trp-His and His-Arg-Trp were reported to block L-type Ca2+ channels [18,19]. Vallabha et al. [11] identified peptides including Leu-Ile, Leu-Ile-Val, Leu-Ile-Val-Thr, and Leu-Ile-Val-Thr-Gln from soybean hydrolysate with ACE inhibitory activity. A series of oligopeptides Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu and Asn–Gly-Pro-Leu-Gln-Ala-Gly-Gln-Pro-Gly-Glu-Arg from squid [20]; Asp-Ser-Gly-Val-Thr, Ile-Glu-Ala-Glu-Gly-Glu, Asp-Ala-Gln-Glu-Lys-Leu-Glu, Glu-Glu-Leu-Asp-Asn-Ala-Leu-Asn, and Val-Pro-Ser-Ile-Asp-Asp-Gln-Glu-Glu-Leu-Met in hydrolysates produced from porcine myofibrillar proteins [12] were found to have antioxidant activity. Other reported peptides were also demonstrated to have physiological activities in preventing lifestyle-related diseases, as summarized in Table 1. Although bioactive peptides from functional foods are less effective than therapeutic drugs by daily intake, peptides must play a crucial role as a natural and safe diet in disease prevention.
Table 1. Reported physiological functions of peptides from food proteins
|
Source
|
Preparation
|
Peptides
|
Action
|
Reference
|
|
Sardine
|
Enzymatic hydrolysis
|
Val-Tyr, Met-Phe, Arg-Tyr, Met-Tyr, Leu-Tyr, Tyr-Leu, Ile-Tyr, Val-Phe, Gly-Arg-Pro, Arg-Phe-His, Ala-Lys-Lys, Arg-Val-Tyr
|
ACE inhibition
|
[6,21]
|
|
Soy bean
|
Enzymatic hydrolysis
|
Leu-Ile, Leu-Ile-Val, Leu-Ile-Val-Thr, Leu-Ile-Val-Thr-Gln
|
ACE inhibition
|
[11]
|
|
Milk
|
Fermentation
|
Ile-Pro-Pro, Val-Pro-Pro
|
Antihypertension
|
[14]
|
|
Buckwheat
|
Pepsin, chymotrypsin, trypsin hydrolysis
|
Val-Lys, Tyr-Gln, Tyr-Gln-Tyr, Pro-Ser-Tyr, Leu-Gly-Ile, Ile-Thr-Phe, Ile-Asn-Ser-Gln
|
ACE inhibitory
|
[22]
|
|
Squid
|
Trypsin hydrolysis
|
Phe-Asp-Ser-Gly-Pro-Ala-Gly-Val-Leu, Asn–Gly-Pro-Leu-Gln-Ala-Gly-Gln-Pro-Gly-Glu-Arg
|
Antioxidation
|
[20]
|
|
Porcine myofibrillar proteins
|
Enzymatic hydrolysis
|
Asp-Ser-Gly-Val-Thr, Ile-Glu-Ala-Glu-Gly-Glu, Asp-Ala-Gln-Glu-Lys-Leu-Glu, Glu-Glu-Leu-Asp-Asn-Ala-Leu-Asn, Val-Pro-Ser-Ile-Asp-Asp-Gln-Glu-Glu-Leu-Met
|
Antioxidation
|
[12]
|
|
Defatted soy protein
|
Thermolase hydrolysis
|
X-Met-Leu-Pro-Ser-Tyr-Ser-Pro-Tyr
|
Anticancer
|
[23]
|
|
Soybean glycinin
|
Enzymatic hydrolysis
|
Leu-Pro-Tyr-Pro-Arg
|
Hypocholesterolemia
|
[24]
|
|
α’ subunit of β-conglycinin
|
Enzymatic hydrolysis
|
Soymetide-13: Met-Ile-Thr-Leu-Ala-Ile-Pro-Val-Asn-Lys-Pro-Gly-Arg
Soymetide-9: Met-Ile-Thr-Leu-Ala-Ile-Pro-Val-Asn
Soymetide-4: Met-Ile-Thr-Leu
|
Immunostimulation; sometide-9 showed the most active in stimulating phagocytosis in vitro
|
[24]
|
|
Soybean conglycinin
|
Protease S hydrolysis
|
Val-Asn-Pro-His-Asp-His-Gln-Asn, Leu-Val-Asn-Pro-His-Asp-His-Gln-Asn, Leu-Leu-Pro-His-His, Leu-Leu-Pro-His-His
|
Antioxidation
|
[25]
|
Many researchers have focused on biologically active peptides present in the sequences of food proteins. Food-derived bioactive peptides as functional foods are expected to be effective in preventing lifestyle-related diseases and maintaining the physical and well-being of humans due to their physiological benefits such as antihypertensive [4–8], antioxidant [9], and anti-inflammatory effects [3]. Industrial manufacturers, thus, need to control the quality and quantity of peptides for the development of functional foods.
    |
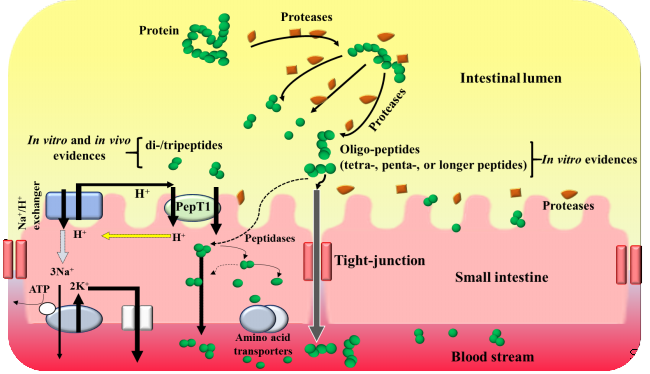 |
| Figure 1. Schematic diagram for peptide absorption in intestinal tract |
The potential physiological effect of a peptide depends on the ability to reach target organ in an active or intact form after oral administration. It was believed that dietary proteins were completely hydrolyzed into their constituent amino acids, and then absorbed into blood via specific amino acid transport systems until the report by Newey and Smyth, who provided the first convincing evidence that dipeptides could be absorbed in intact form. Apart from the aforementioned di-/tripeptides, much work has been focused on the absorption of oligopeptides, since many oligopeptides have been demonstrated to play physiological preventive roles in events against lifestyle-related diseases [13]. In vitro studies reported that oligopeptides could be transported across the brush border membrane. Reecently, Hanh et al. [26] demonstrated for the first time that oligopeptides can be absorbed in vivo in a peptide length-dependent manner of di- > tri- > tetra- > pentapeptide length (Figure 1).
References
[1] D.E.A. Yach, Global burden of chronic diseases: overcoming impediments to prevention and control, J. Am. Med. Assoc. 291 (2004) 2616–2622.
[2] W.C. Willett, J.P. Koplan, R. Nugent, P. Puska, T.A. Gaziano, Disease control priorities in developing countries: Prevention of chronic disease by means of diet and lifestyle changes, D.T. Jamison, J.G. Breman, A.R Measham, G. Alleyne, M. Claeson, D.B Evans, P. Jha, A. Mills, P. Musgrove (Eds.), Disease Control Priorities Project. (2006) 833–850 (Chapter 44).
[3] Y.F. Wang, X. Xu, X. Fan, C. Zhang, Q. Wei, X. Wang, W. Guo, W. Xing, J. Yu, J. L. Yan, H. P. Liang, A cell-penetrating peptide suppresses inflammation by inhibiting NF-κB signaling, Mol. Ther. 19 (2011) 1849–1857.
[4] T. Matsui, M. Sato, M. Tanaka, Y. Yamada, S. Watanabe, Y. Fujimoto, K. Imaizumi, K. Matsumoto, Vasodilating dipeptide Trp-His can prevent atherosclerosis in apo E-deficient mice, Br. J. Nutr. 103 (2010) 309–313.
[5] N. Yamamoto, Antihypertensive peptides derived from food proteins, Biopolymers 43 (1997) 129–134.
[6] T. Kawasaki, E. Seki, K. Osajima, M. Yoshida, K. Asada, T. Matsui, Y. Osajima, Antihypertensive effect of valyl-tyrosine, a short-chain peptide derived from sardine muscle hydrolyzate, on mild hypertensive subjects, J. Hum. Hypertens. 14 (2000) 519–523.
[7] B. Hernández-Ledesma, M. del Mar Contreras, I. Recio, Antihypertensive peptides: production, bioavailability and incorporation into foods, Adv. Colloid Interface Sci. 165 (2011) 23–35.
[7] Y. Hata, M. Yamamoto, M. Ohni, K. Nakajima, Y. Nakamura, T. Takano, A placebo-controlled study of the effect of sour milk on blood pressure in hypertensive subjects, Am. J. Clin. Nutr. 64 (1996) 767–771.
[8] W. Wang, E. Gonzalez De Mejia, A new frontier in soy bioactive peptides that may prevent age-related chronic diseases, Compr. Rev. Food Sci. Food Saf. 4 (2005) 63–78.
[10] Y. Shigemura, M. Nakaba, E. Shiratsuchi, M. Suyama, M. Yamada, T. Kiyono, K. Fukamizu, E.Y. Park, Y. Nakamura, K. Sato, Identification of food-derived elastin peptide, prolyl-glycine (Pro-Gly), in human blood after ingestion of elastin hydrolysate, J. Agric. Food Chem. 60 (2012) 5128–5133.
[11] V. Vallabha, P.K. Tiku, Antihypertensive peptides derived from soy protein by fermentation, Int. J. Pept. Res. Ther. 20 (2013) 161–168.
[12] A. Saiga, S. Tanabe, T. Nishimura, Antioxidant activity of peptides obtained from porcine myofibrillar proteins by protease treatment, J. Agric. Food Chem. 51 (2003) 3661–3667.
[13] N. Yamamoto, Antihypertensive peptides derived from food proteins, Biopolymers 43 (1997) 129–134.
[14] Y. Nakamura, N. Yamamoto, K. Sakai, T. Takano, Antihypertensive effect of sour milk and peptides isolated from it that are inhibitors to angiotensin I-converting enzyme, J. [15] Y. Yamada, K. Nishizawa, M. Yokoo, H. Zhao, K. Onishi, M. Teraishi, S. Utsumi, M. Ishimoto, M. Yoshikawa, Anti-hypertensive activity of genetically modified soybean seeds accumulating novokinin, Peptides 29 (2008) 331–337.
[16] M. Miguel, A. Aleixandre, Antihypertensive peptides derived from egg proteins, J. Nutr. 136 (2006) 1457–1460.
[17] M. Sipola, P. Finckenberg, J. Santisteban, R. Korpela, H. Vapaatalo, M.L. Nurminen, Long-term intake of milk peptides attenuates development of hypertension in spontaneously hypertensive rats, J. Physiol. Pharmacol. 52 (2001) 745–754.
[18] Z. Wang, S. Watanabe, Y. Kobayashi, M. Tanaka, T. Matsui, Trp-His, a vasorelaxant di-peptide, can inhibit extracellular Ca2+ entry to rat vascular smooth muscle cells through blockade of dihydropyridine-like L-type Ca2+ channels, Peptides 31 (2010) 2060–2066.
[19] M. Tanaka, S. Watanabe, Z. Wang, K. Matsumoto, T. Matsui, His-Arg-Trp potently attenuates contracted tension of thoracic aorta of Sprague-Dawley rats through the suppression of extracellular Ca2+ influx, Peptides 30 (2009) 1502–1507.
[20] E. Mendis, N. Rajapakse, H.G. Byun, S.K. Kim, Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects, Life Sci. 77 (2005) 2166–2178.
[21] H. Matsufuji, T. Matsui, E. Seki, K. Osajima, M. Nakashima, Y. Osajima, Angiotensin I -converting enzyme inhibitory peptides in an alkaline protease hydrolyzate derived from sardine muscle, Biosci. Biotech. Biochem. 58 (1994) 2244–2245.
[22] C.H. Li, T. Matsui, K. Matsumoto, R. Yamasaki, T. Kawasaki, Latent production of angiotensin I-converting enzyme inhibitors from buckwheat protein, J. Pept. Sci. 8 (2002) 267–274.
[23] S.E. Kim, H.H. Kim, J.Y. Kim, Y.I. Kang, H.J. Woo, H.J. Lee, Anticancer activity of hydrophobic peptides from soy proteins, Biofactors 12 (2000) 151–155.
[24] M. Yoshikawa, H. Fujita, N. Matoba, Y. Takenaka, Bioactive peptides derived from food proteins preventing lifestyle-related diseases, BioFactors 12 (2000) 143–146.
[25] H.M. Chen, K. Muramoto, F. Yamauchi, Structural analysis of antioxidative peptides from soybean beta-conglycinin, J. Agric. Food Chem. 43 (1995) 574–578.
[26] V.T. Hanh, W. Shen, M. Tanaka, A. Siltari, R. Korpela, T. Matsui, Effect of Aging on the Absorption of Small Peptides in Spontaneously Hypertensive Rats, J. Agric. Food Chem. 65 (2017) 5935-5943.
Vu Thi Hanh – Faculty of food science and technology