1. LUTEOLIN: STRUCTURE AND ORIGIN
Luteolin (Lut) is one of the most common flavonoids, with the scientific name 3',4',5,7-tretrahydroxiflavonone. The molecular formula of Lut is C15H10O6 and the structure is shown in figure 1 [1]. Lut molecules in plants are widely distributed as aglycone molecules without a sugar moiety and as glycoside molecules with a sugar moiety linked to it. Luteolin is weakly soluble in water [2]. Most Lut molecules exist as O-glycosides, with the aglycone linked to the sugar moiety by one or more hydroxyl (OH) groups. The OH groups are located on 5, 7, 3' and 4' positions. Among the sugar moieties, glucose is the major sugar molecule linked to luteolin. In addition, rhamnose, rutinose, arabinose, xylose and glucuronic acid are other sugar derivatives [3,4].
    |
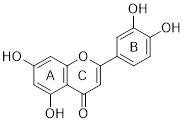 |
| Figure 1: Chemical structure of luteolin |
Luteolin is biosynthesized via the phenylpropanoid pathway. The phenylpropanoid pathway begins with the amino acid phenylalanine, which is the end product of the shikimate pathway. The phenylpropanoid pathway produces a variety of compounds including flavonoids, tannins, and lignin [5]. Luteolin is produced by six enzyme-catalyzed steps in the phenylproponoid biosynthesis (Fig. 2). The first step is the catalytic conversion of phenylalanine to cinnamic acid by the enzyme phenylalanine ammonia-lyase (PAL). Phenylalanine is deamination into cinnamic acid and ammonia. In the second step, cinnamate 4-hydroxylase (C4H) catalyzes the conversion of cinnamic acid to p-coumarate. Then, p-coumarate is converted to p-coumaroyl CoA by the enzyme 4-coumarate CoA-ligase (4CL) [6]. It is then converted to naringenin chalcone (NC) by the enzyme chalcone synthase (CHS), a type III polyketide synthase family enzyme [7]. NC is converted to naringenin, an important compound that plays a key role in the biosynthesis of luteolin by the enzyme chalcone isomerase (CHI) [8]. Furthermore, naringenin is converted to eriodictyol by the enzyme flavonoid 3’-hydroxylase (F3’H), which introduces a hydroxyl group to the 3’ position in the beta ring [9]. Additionally, Lut is formed from naringenin and eriodictyol by flavone synthase (FNS) of the cytochrome P450 family [10, 11].
    |
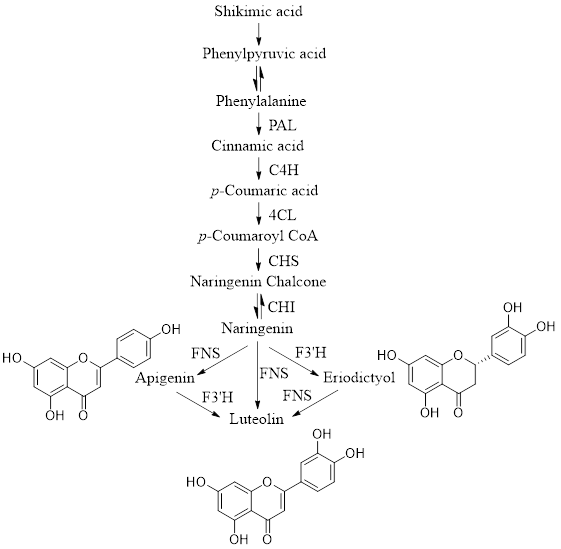 |
| Figure 2: Brief description of the luteolin biosynthetic pathway |
Luteolin is one of the essential phytochemicals in food, found in many vegetables such as broccoli, celery, onion leaves, parsley, carrots, cabbage, peppers and chrysanthemums. For example, among fruits, apple peel is considered a rich source of Lut [12]. Plants rich in Lut are widely used in the production of traditional Chinese medicines for the treatment of inflammatory diseases, cancer and hypertension… [13]. In addition, Lut is also found in a variety of vegetables. Chicory has a Lut content of 37.96 mg/100 g, followed by raw celery with 34.87 mg/100 g Lut. Among herbs, oregano has the highest Lut content of 1028.75 mg/100 g. For fruits and plants, lemon and fresh sage are good sources of Lut with contents of 1.50 and 16.70 mg/100 g, respectively [14]…
2. SOME BIOLOGICAL ACTIVITIES OF LUTEOLIN
Many vegetables, fruits, and herbs such as carrots, cabbage, artichokes, tea, celery, and apples are rich in Lut. Lut is absorbed by the intestinal mucosa, with an average absorption of 0.01–0.20 mg/day (0.0349–0.698 mmol/day) [15]. After oral administration of 14.3 mg/kg Lut, the maximum plasma concentration was 1.97 ± 0.15 g/ml. The time to reach maximum concentration was 1.02 ± 0.22 h, and the half-life of Lut was 4.94 ± 1.2 h. Through intrinsic and extrinsic signaling pathways, Lut is an active compound with antioxidant, tumor suppressor, anti-inflammatory, and anti-apoptotic activities [16,17]. Some of the biological activities of Lut are introduced below.
3.1. Antioxidant activity
Lut and its derivatives are among the flavonoids as antioxidant activity [18]. The antioxidant activity of Lut is associated with C-glycosylation at various sites, which causes the intensity and changes in its antioxidant properties [19]. Pretreatment with Lut (50 mg/kg orally) protected against renal failure through antioxidant detoxification, anti-inflammatory, and anti-apoptotic mechanisms in Wister rats [20]. Carbon tetrachloride (CCl4)-induced hepatotoxicity in a rat model was reduced by the antioxidant properties of Lut by increasing the activities of multiple antioxidant enzymes [21]. Furthermore, the antioxidant properties of Lut have been shown to induce apoptosis through increased antioxidant activity [22]. Lut isolated from Reseda odorata L. alleviates severe acute pancreatitis (SAP) by activating hemeoxigenase-1 (HO−1)-based anti-inflammatory and antioxidant activities through inhibition of nuclear factor kappa B (NF-κB) [23]. During doxorubicin-induced liver and kidney damage during cancer treatment, Lut also acts as a protective molecule as it enhances the therapeutic efficacy of the drug by eliminating the toxic effects of the drug due to its antioxidant nature [24]. Therefore, the effective role of Lut and its glycosides mediates various metabolic processes and acts as a protective molecule by reducing free radicals through its antioxidant capacity.
3.2. Anti-inflammatory activity
Inflammation is the body's normal protective response to damage caused by pathogens. Inflammation occurs when the body's white blood cells increase their activity to protect the body against pathogens. This is a natural process of how the body responds to stimuli with the help of immune cells such as natural killer cells, macrophages, etc. However, inflammation is necessary to reduce the effects of stimuli, which further affect normal cells, but prolonged inflammation affects normal functioning as it leads to chronic conditions, so it needs to be prevented. To control this condition, anti-inflammatory molecules are used to protect cells from side effects [25]. In vitro studies have shown that Lut reduces inflammation caused by oxidized low-density lipoprotein (oxLDL) by inhibiting a signal transducer and activator of transcription 3 (STAT3). Lut interacts with STAT3 primarily through hydrogen bonding [26]. Lut attenuates lung injury by reducing caspase-2-mediated pyroptosis in lung tissue. In a mouse model of acute lung injury (ALI), Lut also modulates regulatory T cell (Treg)-related mechanisms and Treg-derived IL-10, a cytokine that plays an important role in regulating immune responses and maintaining immune homeostasis [27].
3.3. Anticancer activity
Cancer is the most deadly disease in humans, with increasing mortality worldwide due to its incurable nature. The most common cancers are lung, breast, colon, prostate, stomach, liver, ovarian, thyroid, and rectal cancers. The increased incidence of cancer is due to lifestyle changes such as increased tobacco and alcohol consumption, increased body mass index, lack of physical activity, and lower food intake. The Lut molecule has anti-cancer and anti-inflammatory properties [28]. According to in silico analysis, Lut isolated from Tridax procumbens showed high activity and low cardiotoxicity [29]. The Lut molecule has anti-cancer activity due to its antioxidant and free radical scavenging activities [28]. Its effective inhibitory activity against cancer cell proliferation was studied both in vivo at doses ranging from 3 to 50 µM and in vitro at doses ranging from 5 to 10 mg/kg, demonstrating its efficacy [30]. Studies involving human epithelial cancer cells showed that Lut inhibited gastric cancer cells with an IC50 value of 7.1 µg/mL. For lung cancer, Lut was effective with an IC50 value of 11.7 µg/mL and bladder cancer with an IC50 value of 19.5 µg/mL [31]. Leukemia is another major cancer affecting humans, as it produces abnormal white blood cells, causing many deaths. Lut compound also showed inhibitory effects on human leukemia cell lines CEM-C1 and CEM-C7 [32,33]. Lut isolated from Vitex rotundifolia fruits had a growth inhibitory concentration against HL-60, human promyelocytic leukemia cells of 15 µM after 96 h [34,35].
3.4. Anti-diabetic activity
Diabetes is a major health concern worldwide. It is prevalent in both developed and developing countries. According to the 2017 report of the International Diabetes Federation (IDF), nearly 451 million people are affected by the disease, which is expected to increase to 693 million by 2045. Diabetes is one of the leading diseases affecting the health of the world's population and leading to many life-threatening diseases [36]. Lut, a secondary plant metabolite, has been reported to have anti-diabetic properties in many studies. Diabetes affects the myocardium and causes myocardial infarction or damage due to oxidative stress. When treated with Lut, the oxidative stress and the cardiac damage are reduced due to a redirection of the oxidative response through the activation of the sestrin 2-Nrf2-based feedback loop [37]. Long-term diabetes affects cortical neurons; Lut administration significantly ameliorated diabetic conditions, including lipid peroxidation, as it increased in the brain of diabetic mice and also reduced the activities of superoxide dismutase and catalase. This may be explained by the fact that the antioxidant effect of Lut improves neuronal function by reducing neuronal apoptosis, as ChE activity is a result of diabetes, leading to progressive cognitive decline and neurological dysfunction. When diabetic mice were treated with Lut, ChE activity was inhibited, thereby improving the disease state [38]. In silico studies showed that Lut effectively binds to the enzymes alpha-amylase and dipeptidyl peptidase IV (DPP IV). Thus, it prevents glucose uptake and subsequently binds to glutamine-fructose-6-phosphate amido transferase (GFAT1) and Forkhead protein O1 (FOX01), suggesting that Lut may help prevent hyperglycemia. This suggests that Lut is a potent inhibitor of type 2 diabetes [39]. Lut prevents diabetes-induced renal morphological damage [40]. However, further studies are needed to examine the mechanism of Lut's renal protective effect. In another study, compared with untreated cells, Lut significantly increased phosphatidylinositol 3 kinase (PI3K) and insulin receptor substrates 1 and 2 (IRS1/2) in a dose-dependent manner. These findings demonstrate that in adipocytes, insulin sensitivity dependent on the IRSI 1/2 and PI3K pathways was seen. Lut is a noncompetitive inhibitor of alpha-glucosidase enzymes, as it binds to the enzymes at both low and high concentrations. This suggests that Lut has a strong affinity for alpha-glucosidase enzymes [41].
Lut is a phytochemical compound found in almost all plants on earth. Plants containing Lut have been used in many drug delivery systems. The advent of modern analytical techniques has demonstrated the presence of Lut in plants as an important secondary metabolite in various cellular reactions. Meanwhile, Lut is currently being explored for its beneficial activity in various human diseases. It has been clarified that this compound has a potential application in the treatment of various diseases, as it has anti-cancer, anti-inflammatory, anti-diabetic and antioxidant properties. These beneficial activities of Lut have been demonstrated and confirmed in many studies [42].
In the future, the application of Lut and its derivatives with other natural or synthetic drugs in chemoprevention studies can be carried out, as this approach will be more effective in disease control and management. In addition, the bioavailability of Lut can be enhanced by nanoformulation using nanotechnology, which will increase its efficacy when administered to humans and also serve for specific and effective disease management. Currently, several pharmaceutical companies in the world have products such as capsules containing luteolin as a health food with the functions of supporting a healthy brain, supporting the nervous system, and anti-oxidation (Figure 3).
    |
 |
| Figure 3: Image of capsules containing high levels of luteolin compound |
Reference
[1] Weng, C. J., and Yen, G. C. (2012). Flavonoids, a ubiquitous dietary phenolic subclass, exert extensive in vitro anti-invasive and in vivo anti-metastatic activities. Cancer Metastasis Rev. 31, 323–351.
[2] Shakeel, F.; Haq, N.; Alshehri, S.; Ibrahim, M.A.; Elzayat, E.M.; Altamimi, M.A.; Mohsin, K.; Alanazi, F.K.; Alsarra, I.A., (2018). Solubility, Thermodynamic Properties and Solute-Solvent Molecular Interactions of Luteolin in Various Pure Solvents. J. Mol. Liq. 255, 43–50.
[3] Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z., (2021). Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 69, 1441–1454.
[4] Lopez-Lazaro, M., (2009). Distribution and Biological Activities of the Flavonoid Luteolin. Mini-Rev. Med. Chem, 9, 31–59.
[5] Guleria P, Kumar V., (2017). Understanding the phenylpropanoid pathway for agronomical and nutritional improvement of mungbean. J Hortic Sci Biotechnol; 92:335-48.
[6] Wang C, Zhi S, Liu C, Xu F, Zhao A, Wang X, et al., (2016). Characterization and functional analysis of 4-coumarate:coa ligase genes in mulberry. PLoS One; 11:e0155814.
[7] Noel, J.P.; Ferrer, J.-L.; Jez, J.M.; Bowman, M.E.; Dixon, R.A., (1999). Structure of Chalcone Synthase and the Molecular Basis of Plant Polyketide Biosynthesis. Nat. Struct. Biol. 6, 775–784.
[8] Noel, J.P.; Jez, J.M.; Bowman, M.E.; Dixon, R.A., (2000). Structure and Mechanism of the Evolutionarily Unique Plant Enzyme Chalcone Isomerase. Nat. Struct. Biol. 7, 786–791.
[9] CROFT, K.D., (1998). The Chemistry and Biological Effects of Flavonoids and Phenolic Acidsa. Ann. N. Y. Acad. Sci. 854, 435–442.
[10] Martens, S.; Mithöfer, A., (2005). Flavones and Flavone Synthases. Phytochemistry, 66, 2399–2407.
[11] Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al., (2020). Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv, 38, 107316.
[12] Mencherini, T., Picerno, P., Scesa, C., & Aquino, R. (2007). Triterpene, antioxidant, and antimicrobial compounds from Melissa officinalis. Journal of Natural Products, 70(12), 1889–1894.
[13] Selvi, R. B., Swaminathan, A., Chatterjee, S., Shanmugam, M. K., Li, F., Ramakrishnan, G. B., … Kundu, T. K. (2015). Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget, 6(41), 43806.
[14] Lin, Y., Shi, R., Wang, X., & Shen, H.‐M. (2008). Luteolin, a flavonoid with potential for cancer prevention and therapy. Current Cancer Drug Targets, 8(7), 634–646.
[15] Wang, L., Lee, I. M., Zhang, S. M., Blumberg, J. B., Buring, J. E., and Sesso, H. D. (2009). Dietary intake of selected flavonols, flavones, and flavonoid-rich foods and risk of cancer in middle-aged and older women. Am. J. Clin. Nutr. 89, 905–912.
[16] Sun, D. W., Zhang, H. D., Mao, L., Mao, C. F., Chen, W., Cui, M., et al. (2015). Luteolin inhibits breast cancer development and progression in vitro and in vivo by suppressing notch signaling and regulating MiRNAs. Cell. Physiol. Biochem. 37, 1693–1711.
[17] Zhang, B. C., Zhang, C. W., Wang, C., Pan, D. F., Xu, T. D., and Li, D. Y. (2016). Luteolin attenuates foam cell formation and apoptosis in Ox-LDL-stimulated macrophages by enhancing autophagy. Cell. Physiol. Biochem. 39, 2065–2076.
[18] Choi, C.-W.; Jung, H.A.; Kang, S.S.; Choi, J.S. Antioxidant Constituents and a New Triterpenoid Glycoside From Flos Lonicerae. Arch. Pharmacal Res. 2007, 30, 1–7.
[19] Choi, J.S.; Islam, M.N.; Ali, M.Y.; Kim, Y.M.; Park, H.J.; Sohn, H.S.; Jung, H.A. The Effects of C-Glycosylation of Luteolin on Its Antioxidant, Anti-Alzheimer’s Disease, Anti-Diabetic, and Anti-Inflammatory Activities. Arch. Pharmacal Res. 2014, 37, 1354–1363.
[20] lbarakati, A.J.A.; Baty, R.S.; Aljoudi, A.M.; Habotta, O.A.; Elmahallawy, E.K.; Kassab, R.B.; Abdel Moneim, A.E. Luteolin Protects against Lead Acetate-Induced Nephrotoxicity through Antioxidant, Anti-Inflammatory, Anti-Apoptotic, and Nrf2/HO-1 Signaling Pathways. Mol. Biol. Rep. 2020, 47, 2591–2603.
[21] Yan, Y.; Jun, C.; Lu, Y.; Jiangmei, S. Combination of Metformin and Luteolin Synergistically Protects Carbon TetrachlorideInduced Hepatotoxicity: Mechanism Involves Antioxidant, Anti-Inflammatory, Antiapoptotic, and Nrf2/HO-1 Signaling Pathway. BioFactors 2019, 45, 598–606.
[22]. Kang, K.A.; Piao, M.J.; Hyun, Y.J.; Zhen, A.X.; Cho, S.J.; Ahn, M.J.; Yi, J.M.; Hyun, J.W. Luteolin Promotes Apoptotic Cell Death via Upregulation of Nrf2 Expression by DNA Demethylase and the Interaction of Nrf2 with P53 in Human Colon Cancer Cells. Exp. Mol. Med. 2019, 51, 1–14.
[23] Xiong, J.; Wang, K.; Yuan, C.; Xing, R.; Ni, J.; Hu, G.; Chen, F.; Wang, X. Luteolin Protects Mice from Severe Acute Pancreatitis by Exerting HO-1-Mediated Anti-Inflammatory and Antioxidant Effects. Int. J. Mol. Med. 2016, 39, 113–125.
[24] Owumi, S.; Lewu, D.; Arunsi, U.; Oyelere, A. Luteolin Attenuates Doxorubicin-Induced Derangements of Liver and Kidney by Reducing Oxidative and Inflammatory Stress to Suppress apoptosis. Hum. Exp. Toxicol. 2021, 40, 1656–1672.
[25] Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-Inflammatory Effects of Luteolin: A Review of in Vitro, in Vivo, and in Silico Studies. J. Ethnopharmacol. 2018, 225, 342–358.
[26] Ding, X.; Zheng, L.; Yang, B.; Wang, X.; Ying, Y. Luteolin Attenuates Atherosclerosis via Modulating Signal Transducer and Activator of Transcription 3-Mediated Inflammatory Response. Drug Des. Dev. Ther. 2019, 13, 3899–3911.
[27] Zhang, Z.; Zhang, D.; Xie, K.; Wang, C.; Xu, F. Luteolin Activates Tregs to Promote IL-10 Expression and Alleviating Caspase-11- Dependent Pyroptosis in Sepsis-Induced Lung Injury. Int. Immunopharmacol. 2021, 99, 107914.
[28] Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer Activity of the Plant Flavonoid Luteolin against Preclinical Models of Various Cancers and Insights on Different Signalling Mechanisms Modulated. Phytother. Res. 2021, 35, 3509–3532.
[29] Lakhera, S.; Rana, M.; Devlal, K.; Celik, I.; Yadav, R. A Comprehensive Exploration of Pharmacological Properties, Bioactivities and Inhibitory Potentiality of Luteolin from Tridax Procumbens as Anticancer Drug by In-Silico Approach. Struct. Chem. 2022, 33, 703–719.
[30] Kawaii, S.; Tomono, Y.; Katase, E.; Ogawa, K.; Yano, M. Antiproliferative Activity of Flavonoids on Several Cancer Cell Lines. Biosci. Biotechnol. Biochem. 1999, 63, 896–899.
[31] Cherng, J.-M.; Shieh, D.-E.; Chiang, W.; Chang, M.-Y.; Chiang, L.-C. Chemopreventive Effects of Minor Dietary Constituents in Common Foods on Human Cancer Cells. Biosci. Biotechnol. Biochem. 2007, 71, 1500–1504.
[32] Post, J.F.M.; Varma, R.S. Growth Inhibitory Effects of Bioflavonoids and Related Compounds on Human Leukemic CEM-C1 and CEM-C7 Cells. Cancer Lett. 1992, 67, 207–213.
[33] Seelinger, G.; Merfort, I.; Wölfle, U.; Schempp, C. Anti-Carcinogenic Effects of the Flavonoid Luteolin. Molecules 2008, 13, 2628–2651.
[34] Ko, W.G.; Kang, T.H.; Lee, S.J.; Kim, Y.C.; Lee, B.H. Effects of Luteolin on the Inhibition of Proliferation and Induction of Apoptosis in Human Myeloid Leukaemia Cells. Phytother. Res. 2002, 16, 295–298.
[35] Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a Flavonoid with Potential for Cancer Prevention and Therapy. Curr. Cancer Drug Targets 2008, 8, 634–646.
[36] Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.-F. Global, Regional, and National Burden and Trend of Diabetes in 195 Countries and Territories: An Analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790.
[37] Zhou, X.-R.; Ru, X.-C.; Xiao, C.; Pan, J.; Lou, Y.-Y.; Tang, L.-H.; Yang, J.-T.; Qian, L.-B. Sestrin2 Is Involved in the Nrf2-Regulated Antioxidative Signaling Pathway in Luteolin-Induced Prevention of the Diabetic Rat Heart from Ischemia/Reperfusion Injury. Food Funct. 2021, 12, 3562–3571.
[38] Liu, Y.; Tian, X.; Gou, L.; Sun, L.; Ling, X.; Yin, X. Luteolin Attenuates Diabetes-Associated Cognitive Decline in Rats. Brain Res. Bull. 2013, 94, 23–29.
[39] Davella, R.; Mamidala, E. Luteolin: A Potential Multiple Targeted Drug Effectively Inhibits Diabetes Mellitus Protein Targets. J. Pharm. Res. Int. 2021, 33, 161–171.
[40] Wang, Z.; Zeng, M.; Wang, Z.; Qin, F.; Chen, J.; He, Z. Dietary Luteolin: A Narrative Review Focusing on Its Pharmacokinetic Properties and Effects on Glycolipid Metabolism. J. Agric. Food Chem. 2021, 69, 1441–1454.
[41] Wagle, A.; Seong, S.H.; Shrestha, S.; Jung, H.A.; Choi, J.S. Korean Thistle (Cirsium Japonicum Var. Maackii (Maxim.) Matsum.): A Potential Dietary Supplement against Diabetes and Alzheimer’s Disease. Molecules 2019, 24, 649.
[42] Nandakumar Muruganathan, Anand Raj Dhanapal, Venkidasamy Baskar, Pandiyan Muthuramalingam, Dhivya Selvaraj, Husne Aara, Mohamed Zubair Shiek Abdullah and Iyyakkannu Sivanesan. Recent Updates on Source, Biosynthesis, and Therapeutic Potential of Natural Flavonoid Luteolin: A Review. Metabolites 2022, 12, 1145.