Pectin is one of the most important and widely used polysaccharides in the food industry as a gelling agent, stabilizer, coagulant, etc. (Oliveira et al., 2016). Pectin is the methylated ester of polygalacturonic acid, which contains 1,4-linked α-D-galacturonic acid residues (Mesbahi et al., 2005). Pectin can be divided into two types based on the degree of esterification (DE): high-methoxyl pectin (DE > 50%) and low-methoxyl pectin (DE < 50%) (Mesbahi et al., 2005).
Pectin can generally be extracted using various acids: citric acid, oxalic (Koubala et al., 2008), hydrochloric (Kulkarni & Vijanand, 2010), nitric (Constenla, Ponce, & Lozano, 2002), and sulfuric acid (Garna et al., 2007), which are regarded as conventional acids for extraction. Pectin quality depends on extraction time, temperature, and pH (Yapo, 2009).
Pectins are complex heterogeneous polysaccharides. It is used plurally because it differs from its composition from one species to another (the pectin obtained from a citrus differs, e.g., from the apple; even within citrus, there are small differences). Like most other vegetable polysaccharides, it is both polydispersed and polymolecular, and its composition varies with the source and conditions applied during storage. In any pectin sample, parameters such as weight or content of particular subunits will differ from molecule to final molecule (Ruano et al., 2019). Pectins extracted from different raw materials will differ in terms of molecular weight, esterification, methoxyl index... Therefore, they possess different functional properties (Girma et al., 2016).
Biofilm for preserving fresh fruits and vegetables is a worldwide trend due to the increasing human demand for health, food safety and environment (Falguera et al., 2011). Pectin films are notable for their non-toxicity, odorless and biodegradable properties. Furthermore, the coating has low air permeability, thus providing a good barrier to prevent gas exchange (Espitia et al., 2014). The source of pectin is very diverse, but not all types of pectin can make a preservative film. The properties of the film depend on the physicochemical properties of pectin such as esterification, methoxyl index, equivalent, anhydrouronic acid content (Nisar et al., 2018).
The main sources for commercial pectin production are apple pomace and citrus peels (Mesbahi et al., 2005). Meanwhile, passion fruit peel is an abundant and cheap source of raw materials, the amount of this by-product is increasing due to the rapid growth of the planting area and factories processing products from passion fruit. According to the Department of Crop Production, in 2019, the total area of passion fruit nationwide reached about 10,500 ha, the output of fresh fruit reached more than 222,000 tons. Furthermore, according to Tran Hiep et al. (2020), passion fruit peel in Son La province accounts for 41.1% of the total fruit weight. Therefore, taking advantage of the by-product source to extract pectin will both create valuable products for application in the food industry and contribute to solving environmental problems.
    |
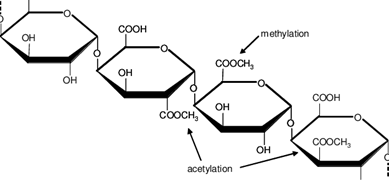 |
| Figure 1. Chemical structure of pectin |
This study aimed to find the optimal conditions for extracting pectin and to develop pectin-alginate (Pec-Alg) composite biofilm based on pectin extracted from purple passion fruit peel. Using a central composite design (CCD) with a response surface methodology (RSM), the results showed that the generated quadratic model adequately explained the data variation and significantly represented the actual relationship between the independent variables and the responses. The study also found the optimal conditions to extract pectin from passion fruit peels as follows: citric axit concentration of 8.00%, temperature of 82.14°C and extraction time of 81.07 min for the highest yield of pectin (24.42%). The pectin extracted under the optimum conditions had the following physicochemical properties: equivalent weight (EW) of 586.92 ± 17.55; degree of esterification (DE) of 38.76 ± 1.52%; methoxyl index (MI) of 3.94 ± 0.13%; total anhydrouronic acid content (AUA) of 76.58 ± 1.19%. These characteristics are suitable for the purpose of edible film making, opening up a potential application for this pectin.
In the next step, pectin extracted under these conditions is used to fabricate pectin-alginate (Pec-Alg) composite biofilm incorporated with alginate, glycerol and Ca2+. Mixing and homogenization methods were used to prepare the Pec-Alg film forming solution. The technical characteristics of the biofilm were determined by the following parameters: Film thickness, tensile strength, elongation at break, water vapor permeability, moisture absorption. Passion fruit samples were coated by dipping method, then preserved under ambient conditions and evaluated the quality change through physiological, mechanical and biochemical parameters. The study found the Pec-Alg biofilm formulation that suitable for preserving passion fruit with the following mixing proportion: pectin:water of 2.5% (w/v), pectin:alginate of 65:35 (v/v), 20% glycerol (w/w) and 5% Ca2+ (w/w). In addition, the specifications of Pec-Alg biofilm were also determined: Thickness of 0.139 ± 0.007 (mm), Tensile strength of 30.84 ± 1.87 (MPa), Elongation at break of 28.23 ± 0.82%, Water vapor permeability of 2.48 ± 0.08 (×10-7g.m-1.h-1.Pa-1), Moisture absorption of 10.03 ± 0.48%. The actual results have shown that Pec-Alg biofilm could maintain the quality and prolong the storage time of fresh passion fruit up to 12 days (4 more days) under normal conditions (ambient temperature range of 29 to 35oC). These results open up potential applications of Pec-Alg biofilm in preserving fresh fruits and vegetables.
References
Constenla D., Ponce A. G., & Lozano J. E. (2002). Effect of pomace drying on apple pectin. LWT-Food Science & Technology. 35: 216–221.
Garna H., Mabon N., Robert C., Cornet C., Nott K., Legros H. & Paquot M. (2007). Effect of extraction conditions on the yield and purity of apple pomace pectin precipitated but not washed by alcohol. Journal of Food Science. 7, C001–C009.
Girma E. & Worku T. (2016). Extraction and characterization of pectin from selected fruit peel waste. International Journal of Scientific Publications. 6(2): 447-454.
Koubala B. B., Mbome L. I., Kansci G., Mbiap T. F., Crepeau M. J., Thibaul J. F., & Ralet M. C. (2008). Physicochemical properties of pectins from ambarella peels (Spondias cytherea) obtained using different extraction conditions. Food Chemistry. 106: 1202–1207.
Kulkarni S. G. & Vijanand P. (2010). Effect of extraction conditions on the quality characteristics of pectin from passion fruit peel (Passiflora edulis f. Flvicarpa L.). LWT- Food Science & Technology. 43: 1026–1031.
Mesbahi G., Jamaliana J., & Farahnaky A. (2005). A comparative study on functional properties of beet and citrus pectins in food systems. Food Hydrocolloids. 19: 731–738.
Nisar T., Wang Z. C., Yang X., Tian Y., Iqbal M., & Guo Y. (2018). Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. International Journal of Biological Macromolecules. 106: 670–680.
Oliveira T. I. S., Redondo L., Moates G. K., Wellner N., Cross K. & Waldron K. W. (2016). Pomegranate peel pectin films as affected by montmorillonite. Food Chemistry. 198: 107–112.
Tran Hiep, Bui Quang Tuan, Le Viet Phuong, Nguyen Hung Son, Le Van Ha & Nguyen Xuan Trach (2020). Passion fruit (Passiflora edulis) peel as feed for ruminants in Vietnam: Quantification, chemical composition and posibility to make silage. Livestock Research for Rural Development. 32 (3), Article #35.
Yapo B. M. (2009). Lemon juice improves the extractability and quality characteristics of pectin from yellow passion fruit by-product as compared with commercial citric acid extractant. Bioresource Technology. 100: 3147–3151.
Thang Nguyen Trong
Department of Postharvest Technology, Faculty of Food Science and Technology