Polyphenols are commonly present as secondary metabolic phytochemicals that naturally occur in plant components, including herbs, fruits, and vegetables. Although they were initially known to be natural pigments in plants, their predominant function is to protect the plant against
environmental stresses, including light oxidation, pathogens, and predators (Bravo, 1998). Polyphenols are made of two or more benzene rings, each having at least one hydroxyl group. More than 8 000 polyphenols have been identified (Tsao, 2010) and are commonly present in human plant-based foods consumed daily worldwide (Bravo, 1998; Scalbert and Williamson, 2000).The scientific interests in polyphenols appear to be because of their physiological potentials in maintaining human health or homeostasis, because the “French paradox”, a reduced incidence of cardiovascular disease (CVD), is associated with healthy “Mediterranean diet” characterized by low consumption of butter and high consumption of vegetables, fruits, cheese, and red wine (or wine polyphenols, like resveratrol) (Renaud and de Lorgeril, 1992; Catalgol et al., 2012). Moreover, epidemiological studies have shown the beneficial effects of polyphenols on human health (MedinaRemon et al., 2017; Tresserra-Rimbau et al., 2014).
Clinical trials showed that an increased intake of dietary flavonoids, particularly flavanones, was associated with a significantdecrease in postprandial lipid response (triglycerides and cholesterol) in the circulating bloodstream (Vetrani et al., 2018). A clinical report by Vabeiryureilai and Lalrinzuali (2015) indicated the protective effect of polyphenols against vessel dysfunction by a daily intake of hesperidin (500 mg/day) for 3 weeks, in which an increase in nitrogen monoxide (NO) production and a decrease in circulating inflammatory biomarkers was observed. Moreover, it is well established that polyphenols (in particular, flavonoids) exhibit beneficial effects against the development of chronic degenerative diseases, such as diabetes, hypertension, and lipidemia in human studies. For every bioactive compound showed many bioactivities, the efficiency of its physiological functions must be determined by the bioavailability of polyphenols (Scalbert and Williamson, 2000; Wang, et al., 2017), but the understanding of their fate after intake is still insufficient and needs to be further investigated. Furthermore, while the functions of absorbed substances primarily depend on their bioavailability, their metabolism during absorption process is also crucial for the compound’s physiological activity (Williamson et al., 2018). Indeed, the number and specific positions of the hydroxyl groups on the flavanones’ aromatic rings have a great influence on their physiological effects (Barreca et al., 2017). However, further inquiries regarding the metabolites of polyphenols as well as their bioavailability are needed. Although mono-phenolic acids, such as chlorogenic acid and ferulic acid, are out of the category of polyphenols, this will also be discussed their bioavailability because of common naturally occurring phenols.
Tissue accumulation of polyphenols: Polyphenol-induced health benefits, such as improved
insulin sensitivity (Park et al., 2012), vasorelaxation (Lorenz et al., 2009), and anti-atherosclerosis (Loke et al., 2010) indicate their local action or accumulation in the organs. However, studies that report tissue accumulation of polyphenols in the circulatory system are rare.
Non-absorbable polyphenols: Spinosin, a flavonoid glycoside that performs sedation and hypnosis actions, was incorporated mainly via MCT and partly SGLT1, together with recognition by P-gp-efflux route (Meng et al., 2016). In contrast, tomatoside A, a steroidal saponin from tomato seed (Li et al., 2018), as well as condensed catechins, theaflavins (Takeda et al., 2013), could not cross the intestinal membrane (Fig. 2). However, the saponin could reduce glucose transport by suppressing the expression of GLUT2. In in vitro transport experiments using Caco-2 cells, which are derived from human colon carcinoma, the saponin could be incorporated into cells via ASBT and then pumped out to the apical side through the MRP2 efflux route. During the influx/efflux transportation process, intracellular protein kinase C (PKC) was activated to inhibit GLUT2 expression (Li et al., 2018). For the intestinal transportation of theaflavins as non-absorbable compounds, like tomato saponin, more evidence on transportation behavior was visually obtained using MALDI-MS imaging technique, which suggested that theaflavins were incorporated into rat intestinal cells through both MCT and OATP transporters (Fig. 2) (Nguyen et al., 2019). Non-targeted MALDI-MS analysis also revealed that there was an efflux of non-absorbable theaflavins back to the gut via the ABC transporters, like other polyphenols (Chan et al., 2007), without any MS detection of metabolites.
    |
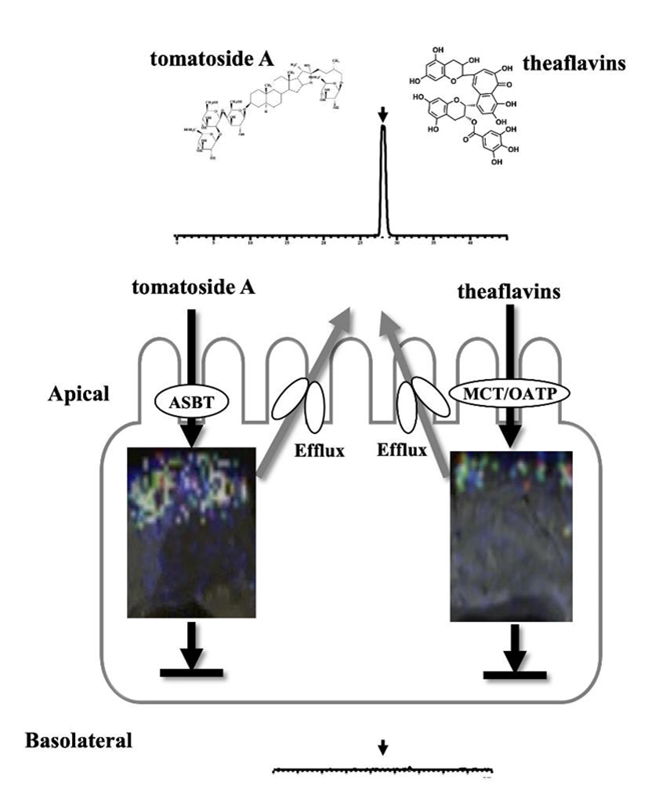 |
| Fig. 2. Visualized absorption behavior of steroidal saponin (tomatoside A) and theaflavins across rat intestinal membrane through matrix-assisted laser desorption/ionization-mass spectrometry (MALDI-MS) imaging |
Polyphenols have diverse physiological potentials for maintaining homeostasis. However, these compounds are susceptible to metabolic phase I/II reactions during intestinal absorption and form a variety of conjugates. Although in this review, the bioavailability of major polyphenols was discussed, little is known about the absorbability of other naturally occurring polyphenols. Considering the in vivo findings that polyphenols can provide diverse health benefits, more research on tissue distribution is required to explore the effects of polyphenols. Furthermore, the combinatory effect of polyphenols on contaminated food compounds should also be taken into consideration while developing strategies for polyphenol-derived health promotion (Wendling et al., 2015).
References:
Abdelkawy, K.S., Balyshev, M.E., and Elbarbry, F. (2017). A new validated HPLC method for the determination of quercetin: Application to study pharmacokinetics in rats: A new HPLC assay and pharmacokinetic analysis of quercetin. Biomed. Chromatogr., 31, e3819
Boocock, D.J., Faust, G.E.S., Patel, K.R., Schinas, A.M., Brown, V.A., Ducharme, M.P., Booth, T.D., Crowell, J.A., Pertoff, M., Gescher, A.J., Steward, W.P., and Brenner, D. (2007). Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomarkers & Prevention, 16, 1246–1252.
Booth, A.N., Jones, F.T., and deEds, F. (1958). Metabolic fate of hesperidin, eriodictyol, homoeriodictyol, and diosmin. J. Biol. Chem., 230, 661–668.
Brasnyó, P., Molnár, G.A., Mohás, M., Markó, L., Laczy, B., Cseh, J., Mikolás, E., Szijártó, I.A., Mérei, Á., Halmai, R., Mészáros, L. G., Sümegi, B., and Wittmann, I. (2011). Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr., 106, 383–389.
Bravo, L. (1998). Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev., 56, 317–333.
Matsui T. (2021). Polyphenols-absorption and occurrence in the body system. Food Sci. Technol. 28 (1), 13–33.
Nguyen, H.N., Tanaka, M., Komabayashi, G., and Matsui, T. (2016). The photobase generator nifedipine as a novel matrix for the detection of polyphenols in matrix-assisted laser desorption/ionization mass spectrometry. J. Mass Spectr., 51, 748–756.
Nielsen, S.E., Breinholt, V., Justesen, U., Cornett, C., and Dragsted, L.O. (1998). In vitro biotransformation of flavonoids by rat liver microsomes. Xenobiotica, 28, 389–401.
Nishijima, T., Iwai, K., Saito, Y., Takida, Y., and Matsue, H. (2009). Chronic ingestion of apple pectin can enhance the absorption of quercetin. J. Agric. Food Chem., 57, 2583– 2587.
Ouyang, Z., Zhao, M., Tang, J., and Pan, L. (2012). In vivo pharmacokinetic comparisons of ferulic acid and puerarin after oral administration of monomer, medicinal substance aqueous extract and Nao-De-Sheng to rats. Pharmacognosy Magazine, 8, 256–262.
Vu Thi Hanh – Faculty of Food Science and Technology